К слову, если вам нужен VPS для хранения данных, здесь можно найти услугу VPS
На примере сервиса Ucaller, предоставляющего такой сервис, можно увидеть, что доставлять коды можно двумя
Profit Server уже почти 10 лет работает в сфере предоставления услуг по аренде выделенных
Ищете хостинг или виртуальный сервер с комфортными условиями по оплате и использованию? Такие решения
IP-телефония — современный способ коммуникации с клиентами, предпочитающими вести обсуждения голосом с живым оператором,
Перечень операций в рамках настройки 1С и стоимость услуг зависит от специфики компании, внедряемого/настраиваемого
При заказе VPS/VDS от CloudVPS можно воспользоваться готовыми дистрибутивами для быстрого старта.
JUSTHOST — провайдер услуг, у которого есть все необходимое для уверенного присутствия в интернете.
Согласно статистике ежегодно российские маркетплейсы растут x2. Это касается, как заработка самих площадок, так
Regfon, как комплексное решение, включает услуги по продаже/аренде виртуальных номеров, ВАТС и оборудования для
Ниже — обзор популярных сервисов авторизации по звонку, с помощью которых вы сможете быстро
Ремонт в стиле лофт - это не просто модное направление в дизайне, это целая
Обзор модельного ряда торцовочных пил JET: стоимость, преимущества, недостатки и отзывы владельцев.
Обзор и технические характеристики. Отзывы и рейтинг реальных пользователей. Достоинства, недостатки, комментарии.
⭐Представляем рейтинг электросамокатов 2021. 👍Лучшие 16 моделей, которые были отобраны нашими экспертами по отзывам
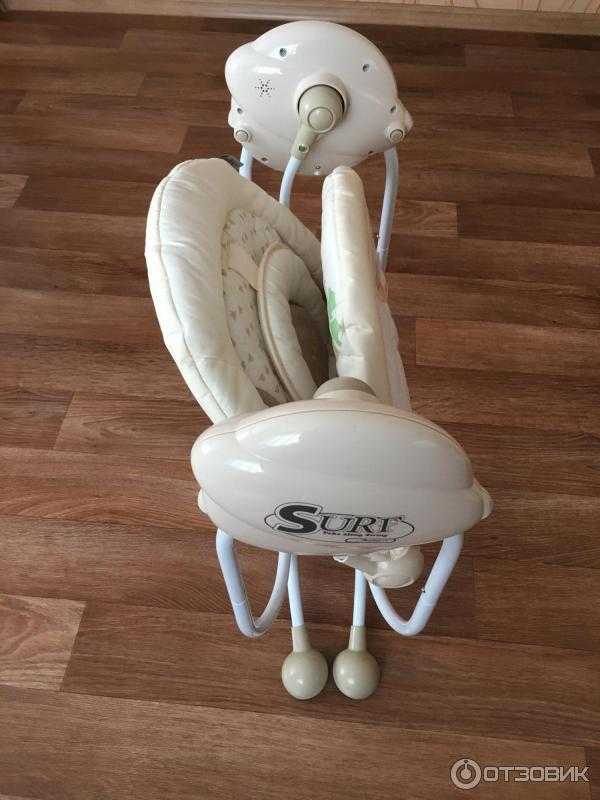
Детские коляски Jetem. Коляска трость Jetem. Прогулочная коляска Jetem. Коляска трансформер Jetem. Цена детских
Обзор и технические характеристики. Отзывы и рейтинг реальных пользователей. Достоинства, недостатки, комментарии.
В Сайдексе Беговел JETCAT 14 Race Pro - купить , скидки, цена, отзывы, обзор,
Подробный ТОП-10 Лучших Моделей 2020 года хороших ТВ боксов. Мы знаем какая Лучшая Смарт
В чем разница между Samsung Galaxy Buds и JBL Under Armour True Wireless Flash?
Обзор и технические характеристики. Отзывы и рейтинг реальных пользователей. Достоинства, недостатки, комментарии.
JBL T110BT предлагают отличное звучание басов и подходят для прослушивания разнообразных жанров музыки.
Самые лучшие портативные колонки JBL в рейтинге ТОП-10 2021 года🎧 Какие Bluetooth колонки JBL
Принято считать, что готовые корпусные сабвуферы – удел новичков, не претендующих на нечто особенное
Обзор и технические характеристики. Отзывы и рейтинг реальных пользователей. Достоинства, недостатки, комментарии.
Обзор JBL Tune 750BTNC 2020 года 🎧 Беспроводные Bluetooth наушники с отличным звуком, ANC
Рейтинг 10 лучших колонок jbl. Советы по выбору, характеристики и отзывы представленных моделей.
Мало кто сейчас не знаком с брендом JBL из семейства HARMAN — за несколько
Всем привет. Сегодня я расскажу вам о музыкальной системе, а именно о саундбаре JBL
ТОП 10 главных новинок от JBL на 2021, портативная колонка JBL Charge 5, TWS